Introduction to marine iron cycle
- Fe Abundance
- Fe residence time
- Redox states
- Ligand
- Solubility
- Source and sink
- Summary: the fate of iron
- References
Fe Abundance
Fe is the 4th most abundant element in the Earth’s crust, but very rare in the oceans.
Fe residence time
100-200 years
Redox states
Iron in ocean exist mostly in Fe (III) (“ferric”), because Fe(II) (“ferrous”) tends to be oxidized to Fe(III).
Fe(III) tends to hydrolyse and produce Fe(OH)3 that immediately converts to polymeric hydroxide (聚合氧化物) and precipitate out of solution. Therefore, the pH also influences the Fe solubility. $$ \ce{Fe3+ + 2H2O = FeO(OH) + H+} $$
Ligand
The structure of Fe(III) makes it acceptable of a large variety of ligands to form coordination complexes. Various cheltating compounds cause iron oxide-hydroxide (like rust) to dissolve even at neutral pH.
Complex:配体,也叫络合物
Chelation:螯合物:是配合物的一种
Ligand-iron complex is solubable
As above said, the presence of organic iron-binding ligands stabilizes (or “buffers”) dissolved iron against oxy-hydroxide formation and precipitation. And nearly all of the dissolved iron in seawater is bound to natural ligands.
Strong and weak ligand
The ‘strong’ iron-binding ligand class (L1) is confined to the upper ocean, produced by heterotrophic and autotrophic bacteria. L1 class may be composed largely of siderophores
The ‘weak’ iron-binding ligand class (L2) is generally observed throughout the water column, released during the decomposition of organic matter.
Solubility
The essence of solubility is size, and the size partitioning is operational. The solubility of Fe as Fe(III) is low but is promoted by ligands, despite that the particle however like scavenging Fe.
Dissolved iron (dFe, < 0.40μm) :
99% of dissolved iron is bound to organic ligands throughout the world’s oceans
soluble components (sFe, < 0.020 μm)
colloidal component (cFe, 0.02-0.4 μm): chelated with organic ligands.
Colloid:胶体,分散系的一种。胶体不一定是配合物,两者无必然联系。
Particle (pFe, > 0.40μm)
Source and sink
Source
sediment, hydrothermal vent, dust
Sink
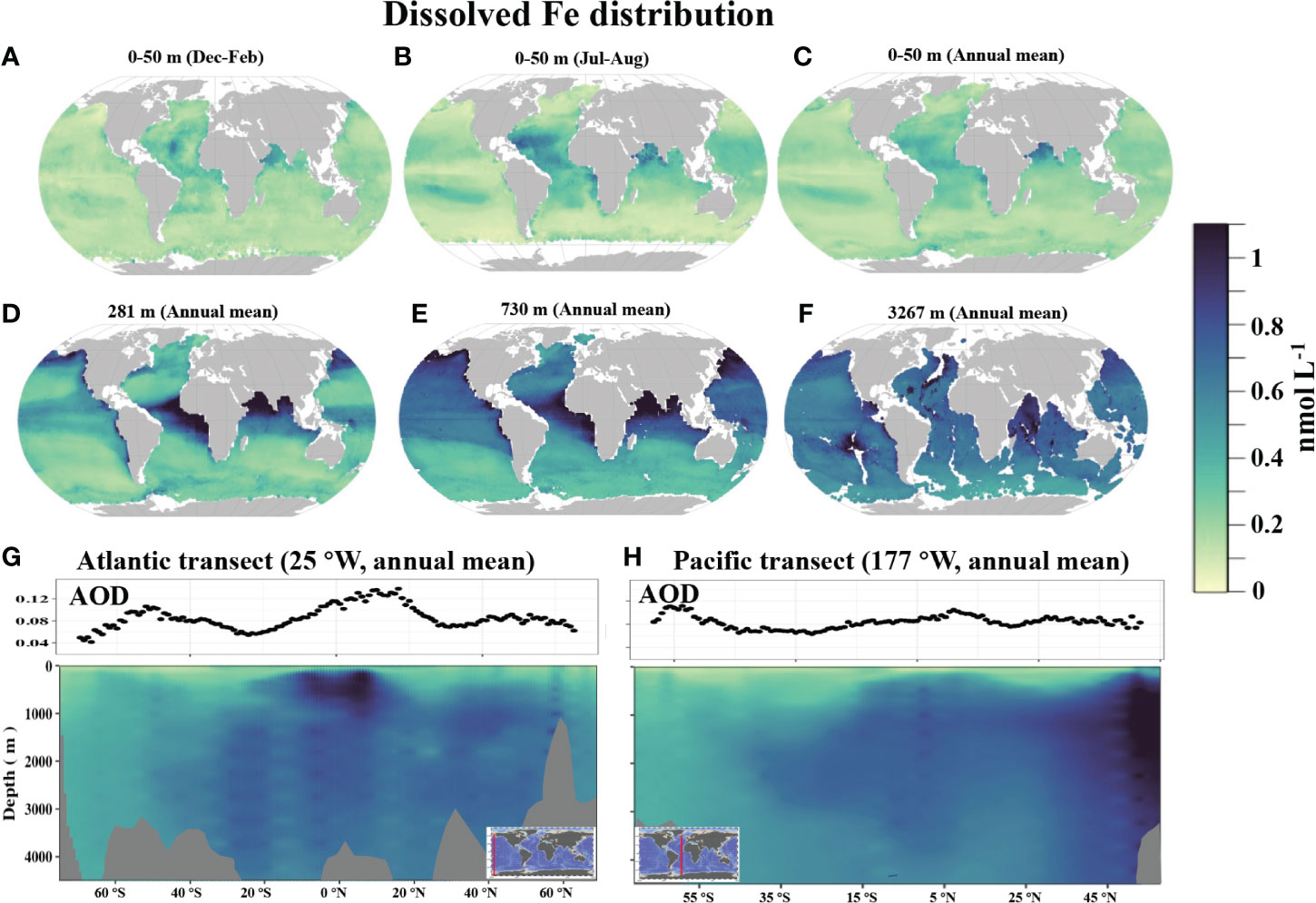
Scavenging out of water column: adsorption, precipitation and aggregation processes convert soluble iron into colloidal or particulate forms. Older waters have stronger scavenging loss, and less dFe. See below showing the difference between old Pacific and young Atlantic (Huang et al. 2022).
Internal
Preform and regenerated as other tracers
Summary: the fate of iron
This is mostly from Boyd & Ellwood 2010
All processes
Dust deposition $\rightarrow$ dissolution of aerosol iron and interact with ligand (within the soluble pool) $\rightarrow$ formation of complexes FeL (in the colloid pool) $\rightarrow$ Lithogenic particles settle out -> remineralisation
Acquisition by plankton
Iron acquisition strategies of planktons include: siderophore-mediated uptake (L, siderophores are chelators released by bacteria), direct colloidal iron uptake (C) and soluble iron uptake (T). The latter two dissoved Fe species are used by eukaryotes like diatoms before reduced by enzymes presented in membranes.
Reduction of Fe: transform Fe(III) to Fe(II)
The organic particulate Fe (from carcass) then will be rapidly recyled by microzooplankton/bacteria etc.
Water column: remineralisation vs. scavenging
< 250m: Scav = Remin
250-1000m: Remin > Scav, increasing dFe
$>$ 1000 m: Remin < Scav, decreasing dFe
References
Boyd, P. W., & Ellwood, M. J. (2010). The biogeochemical cycle of iron in the ocean. Nature Geoscience, 3(10), 675-682.
Tagliabue, A., Bowie, A. R., Boyd, P. W., Buck, K. N., Johnson, K. S., & Saito, M. A. (2017). The integral role of iron in ocean biogeochemistry. Nature, 543(7643), 51-59.
Gledhill, M., & Buck, K. N. (2012). The organic complexation of iron in the marine environment: a review. Frontiers in microbiology, 3, 69.
Huang, Y., Tagliabue, A., & Cassar, N. (2022). Data-Driven Modeling of Dissolved Iron in the Global Ocean. Frontiers in Marine Science, 9.